Liquid Crystal Nanoparticles (LCNP) Enhances Antimicrobial Efficacy
KEY INFORMATION
Healthcare - Pharmaceuticals & Therapeutics
TECHNOLOGY OVERVIEW
Chronic infections (lungs, wounds, eyes) are typically due to bacterial biofilm infections and are untreatable with commercially available antibiotics. In up to 80% of patients with Pseudomonas aeruginosa infection, there will be no response to antibiotics.
Currently for P. aeruginosa, aminoglycoside and colistin are mainstay therapy. Therapy can be lifelong with the goal of keeping the infection at bay, not focused on eradication. New formulation techniques are focusing on liposomal formulations; however, these formulations have stability, manufacturing and effectivity issues that are restricting their entry into the market.
The technology comprises a novel formulation approach that direct antibiotics into biofilm (chronic) infections, enhancing the efficacy of cationic antimicrobials by at least 100-fold against biofilm bacterial infections.
The technology owner is seeking licencing, co-development opportunities as well as contract research partnership with parties interested in this novel antimicrobial formulation.
TECHNOLOGY FEATURES & SPECIFICATIONS
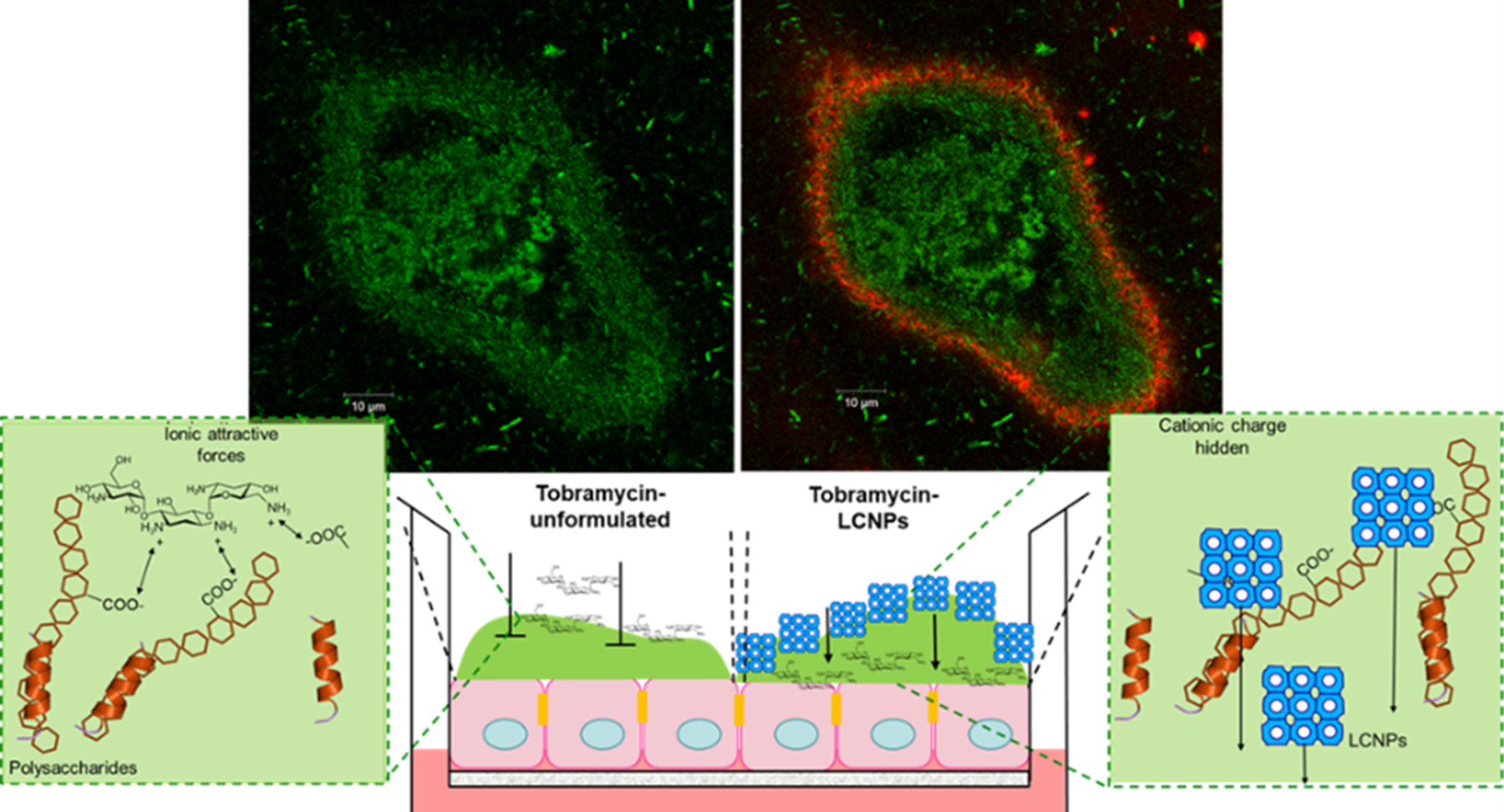
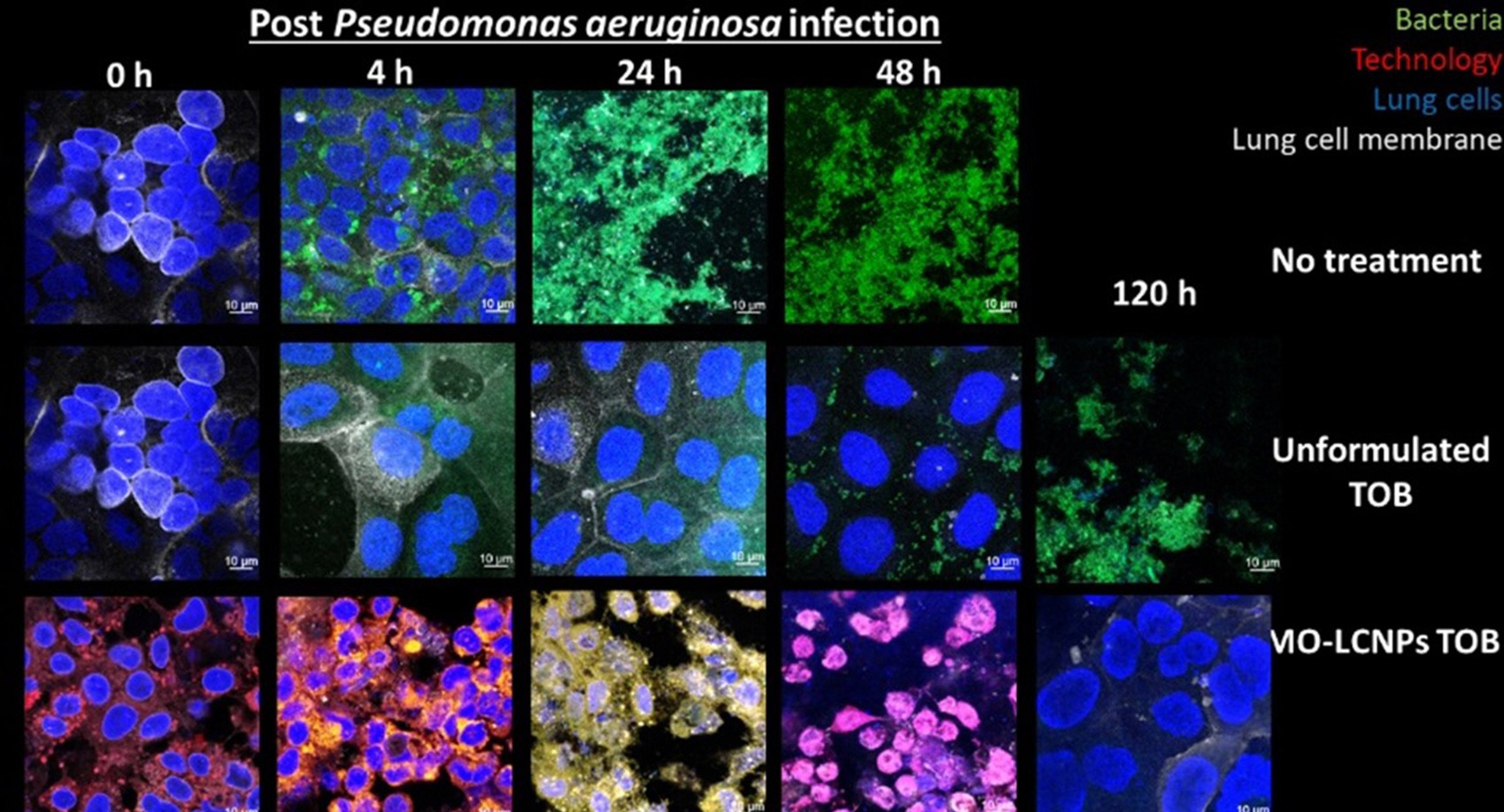
Liquid Crystal Nanoparticles (LCNPs) (blue cubes in schematic) are lipids swollen in water, composed of Generally Recognised as Safe (GRAS) ingredients. In comparison to unformulated and liposomal tobramycin formulations, LCNPs can improve the penetration of the antibiotics across the biofilm and bacterial cellular membrane, improving the overall antimicrobial effect. In advanced human based cellular cultures of cystic fibrotic lung cells infected with P. aeruginosa biofilms, the LCNPs containing tobramycin eradicated the infection within two days and maintained a healthy, uninfected epithelium after stopping the treatment. Tobramycin (unformulated) could not eradicate the infection and once therapy was stopped, resulted in the lung cell culture dying.
The LCNPs can also enhance the antimicrobial activity of all aminoglycoside and other cationic antibiotics, like colistin. Which can be used to treat other local infections, such as wounds, eyes and ear infections.
POTENTIAL APPLICATIONS
The primary market for the Liquid Crystal Nanoparticles Technology is pharmaceutical companies that are interested in reformulation of cationic antimicrobials.
Unique Value Proposition
- Platform technology, suitable for multiple antimicrobials
- Improve the efficacy of cationic antibiotics by up to 100 000-fold (at least 100-fold)
- Suitable for cystic fibrosis lung infections (inhalable), chronic wounds, eye and ear (topical) Pseudomonas aeruginosa biofilm infections
- Lowers dose required of antibiotic while ensuring effectiveness, safety and bacterial eradication is achieved with this platform technology
- Can eradicate biofilm bacterial infections after two doses, while currently available formulations cannot eradicate infections
