3D-Printed Titanium Keratoprosthesis: A Toothless Solution for Severe Corneal Blindness
KEY INFORMATION
TECHNOLOGY OVERVIEW
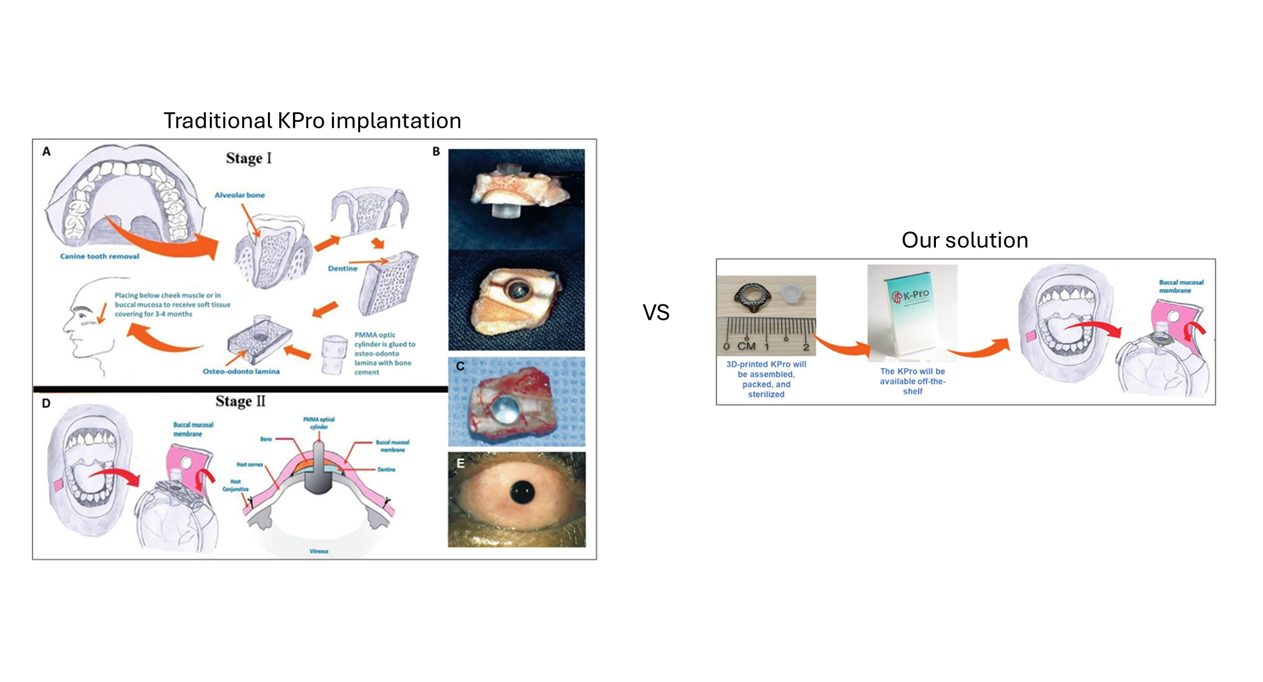
A next-generation, fully synthetic keratoprosthesis (KPro) has been developed to address severe corneal blindness in patients unsuitable for conventional corneal transplantation or existing KPros. Traditional osteo-odonto-keratoprosthesis (OOKP, “tooth-in-eye”) remains effective but is surgically complex, costly, and requires removal of a healthy tooth.
This innovation replaces autologous tissue with a 3D-printed Ti6Al4V titanium lattice skirt engineered for optimal biointegration, paired with a polymethylmethacrylate (PMMA) optical cylinder secured by a proprietary mechanical locking system.
The device is off-the-shelf, sterilised, and designed for single-stage implantation beneath a buccal mucosa graft, significantly reducing patient burden and simplifying logistics. Preclinical rabbit studies demonstrated excellent biocompatibility and stable tissue integration. The procedure eliminates the need for dental/maxillofacial surgeons and long waiting periods, making it particularly valuable in low-resource settings.
The ideal partners for this technology include medical device companies specialising in ophthalmology or surgical implants, contract manufacturers with ISO 13485 certification, and 3D printing companies experienced in producing medical-grade titanium components. Collaboration with such partners will support further development, regulatory approval, and commercial scaling of this next-generation keratoprosthesis for global deployment.
TECHNOLOGY FEATURES & SPECIFICATIONS
- 3D-Printed Ti6Al4V Titanium Skirt
- Porous lattice design for fibrovascular ingrowth and secure ocular fixation.
- PMMA Optical Cylinder
- High-clarity visual channel with a proprietary locking mechanism—no adhesives required.
- Surgical Advantages
- One-stage procedure, no tooth harvesting, reduced operative time, and improved reproducibility.
- Manufacturing & Deployment
- Additive manufacturing enables consistent quality and scalability.
- Supplied sterilised and ready for implantation.
POTENTIAL APPLICATIONS
- Primary Use: Treatment of severe corneal blindness due to multiple graft failures, trachoma, chemical burns, cicatricial conjunctivitis, and ocular graft-versus-host disease
- Replacement For: OOKP, Boston KPro Type II, and MICOF devices in patients with poor dental health or limited donor cornea availability.
- Cross-Disciplinary Potential: Platform technology for other 3D-printed implants in orthopaedics and craniofacial reconstruction.
Unique Value Proposition
- First fully synthetic KPro designed to replace OOKP.
- Eliminates need for donor or autologous tissue, avoiding tooth resorption and immune rejection risks.
- Single-stage surgery reduces patient morbidity, cost, and recovery time.
- High reproducibility and global accessibility through additive manufacturing.
- Off-the-shelf availability supports rapid deployment, including in resource-limited settings.
This innovation offers a synthetic, cost-effective, accessible alternative optimised for extreme ocular surface disease.
